
The pandemic has made us into instant virus experts. A quick online search tells me that on the day I write this, some 460,000 people are testing positive around the world for Covid-19, a total of 240 million have caught the virus since March 2020 and almost five million have died.
Shattering enough for us humans, but insignificant as a snapshot of the totality of life on earth. Consider that, every second, 1025 viruses – a 25 digit number – infect a host cell across all species. Or that there are 1031 viruses on the planet, ten times more than all the animals, plants and bacteria added together, a number greater than all the stars in the universe.
These are the kind of facts you learn from Michael Cordingley, an academic and pharma industry consultant whose book was published four years before Covid arrived. Back then it would have been a harder sell to interest people in viruses, hence the subtitle. Even more so since Cordingley’s exposition of molecular biology and population genetics is not always easy to follow. Today the relevance of the subtitle is obvious: if not a living thing in the strict sense, Covid is certainly an agent. Whether jumping across the species barrier from animals to humans, or producing ever more effective variants, the virus has its own self-perpetuating agenda.
Ever since genetic material first appeared inside cells in primeval seas about 3.5 billion years ago, the start of a long road towards specialisation and complexity, some of that genetic material worked out a means of piggybacking cellular organisms without the bother of independent living. As soon as viruses appeared, organisms evolved defences against them, launching a three billion year arms race between hosts and invaders.
As Cordingley explains, ‘parasite’ is only one point along a spectrum of terms defined according to how much a virus harms or benefits its hosts, and the outcome of the evolutionary arms race determines which point is ultimately selected.
At the simplest level, a virus is a killing machine: it invades a cell, hijacks it to produce copies of itself, and then ‘lyses’ or dissolves the cell to spread the infection to other cells. Seeing this happen inside their Covid patients was a harrowing experience for intensive care doctors during the pandemic.
And yet, the mass murder of hosts turns out to be a poor long-term strategy for a virus dependent on such hosts.
Starting with bacterial viruses or phages (which still account for the majority of viruses today), alternative strategies emerged where viruses learned to switch off their virulence, buying time for their hosts to reproduce naturally. With their own evolutionary destiny tied to their hosts’, it paid off to help these hosts survive against their competitors.
As Cordingley explains, none of this would have been possible without viruses’ key advantage in the arms race. Those composed of RNA rather than error-correcting DNA can randomly mutate tens of thousands of times faster than their hosts can evolve by reproduction. That allows viruses to explore a much larger portion of ‘evolutionary space’, in the process hitting on more inventive solutions.
That’s generally bad news for virus hosts, where the shape-shifting abilities of pathogens such as influenza or HIV-1 enable them to constantly outwit human immune systems. Their genetic plasticity also makes viruses good at jumping from species to another. Cordingley offers us the visual metaphor of the ‘fitness landscape’, where the infamous R0parameter is the height dimension, and horizontal dimensions measure the genetic distance between viral strains1)There’s some mathematical heavy lifting behind the scenes here: a strand of viral RNA comprised of 1000 amino acid bases would be represented as a point in a 1000-dimensional space, which would have to be collapsed into a functional space of far fewer dimensions.
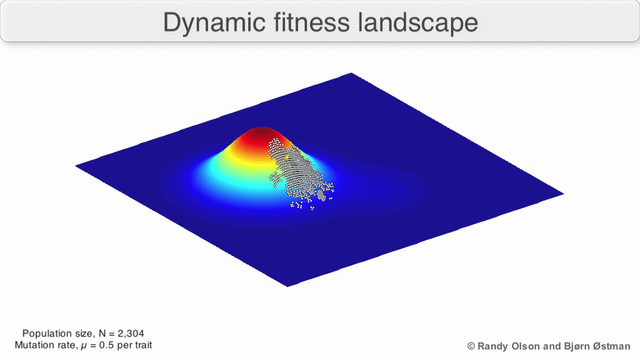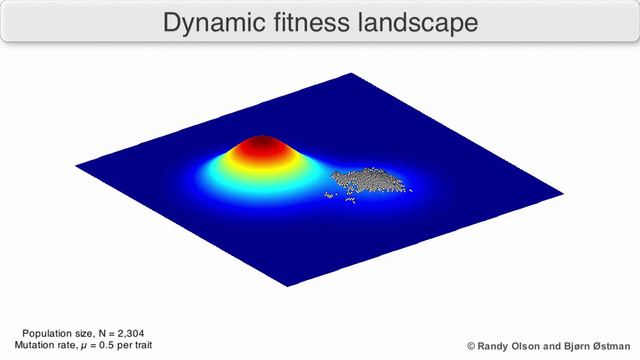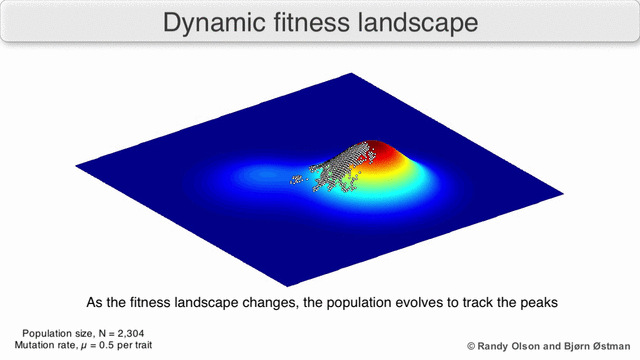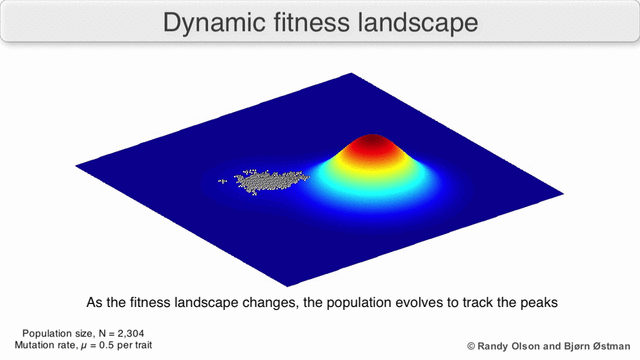
As viruses mutate, they roam around this landscape. Wherever R0 is less than zero, the landscape is underwater; it means infections always fizzle out. But if two species are connected by a strip of land above the R0 = 0 waterline, then viruses can jump the barrier. This unseen proximity to other species can be heightened by environmental changes or human activity, and is an essential part of understanding viruses.
Over the passage of evolutionary time, some animals have learned to accommodate viral invaders, which makes them ‘reservoir species’, primed to infect others. For example bats have particularly high body temperatures associated with flying, which mimics the effect of fever in fighting off viral attacks.
But things don’t go well when bat viruses colonise new species. A decade ago, evidence first emerged that such crossover infections made human immune systems go haywire, causing more damage than the virus itself. Was this related to the now notorious cytokine storm that killed so many Covid victims? Unfortunately, we still don’t know the host species that harboured Covid’s precursor, and we may never know.
However, every now and again (in evolutionary terms), viral inventiveness pays off for the hosts as well.
Witness the example of so-called endogenous retroviruses (ERVs), which like HIV manage to inject themselves into their host cell DNA, but go further and do this in the sperm or egg cells of their hosts. They then become what Cordingley refers to as the hosts’ ‘viral heritage’, inherited by the hosts’ offspring.
This may have happened tens of millions of years ago when early mammals stopped laying eggs and started producing live young. The problem with live birth is that the mother’s immune system is primed to attack an embryo that doesn’t share her genetic material. An ERV may have invaded the mother and managed to suppress her immune system sufficient to allow pregnancy. In other words, mammalian placentas were ‘invented’ by a domesticated virus.
According to research cited by Cordingley, as much as 40% of our own genomes consists of so-called ‘mobile genetic elements’, or the fossils of viruses absorbed long ago. Although inactive today, they improved the fitness of our ancestors, hence their survival.
But there would have been a high price paid for such genetic improvements – the decimation or near-extinction of a species prior to its reaching an accommodation with a new virus. As Cordingley acknowledges, this price is simply too high for humans. Fortunately, our culture and ability to change behaviour gives us an advantage against viruses that other animals don’t have.
As a scientist, he is positive about the role of science in beating viruses such as smallpox and HIV-1. He dismisses as an overreaction the outcry against so-called gain-of-function (GOF) experiments that made certain flu viruses more transmissible between animals, and led to a US government ban on the practice in 2014.
Four years after the book’s publication, readers might view the crowning scientific achievement as being the rapid creation of vaccines that protected millions against Covid, in particular the breakthrough use of new messenger RNA technology. They also might view it as entirely plausible that a country with lower ethical and biosafety standards such as China could have conducted GIF research that led to Covid escaping from a Wuhan laboratory.
The world in 2021 is utterly different to 2017, but it takes this pre-pandemic book to make sense of it.
References
| 1. | ↑ | There’s some mathematical heavy lifting behind the scenes here: a strand of viral RNA comprised of 1000 amino acid bases would be represented as a point in a 1000-dimensional space, which would have to be collapsed into a functional space of far fewer dimensions |
 Levelling the Playing Field
Levelling the Playing Field
 Barclays and Labour's growth plan
Barclays and Labour's growth plan
 Plummeting bonds reflect souring UK mood for outsourcing and privatisation
Plummeting bonds reflect souring UK mood for outsourcing and privatisation
 Dimon rolls trading dice with excess capital
Dimon rolls trading dice with excess capital